You are here
Drive Off Moisture and Get Better Welds
Beware of electrode coatings with more than .20% moisture. Welding releases hydrogen, hydrogen promotes cracking...
Welding electrode coatings, especially low hydrogen electrodes, readily attract moisture in the coating. This moisture is a major cause of weld cracking and porosity. While plain water itself wouldn't damage the weld, the heat and arc break down water into its basic elements, hydrogen and oxygen. These elements in or near the molten weld are defect carriers that pose a significant threat to the quality of the weld. In addition to water, there are other hydrogen contamination sources such as oil and grease, dirt and coatings.
Atomic hydrogen produced from the moisture at the arc diffuses in the weld, goes into solution and settles in the atomic structural voids. If the metal cools rapidly enough, not all the hydrogen is absorbed. Some migrates to the heat-affected zone of the parent metal, some forms gas pockets or evaporates and some helps form other undesirable impurities in the weld.
Steel's ability to absorb hydrogen increases with temperature. Molten steel absorbs more than .0024% hydrogen, and at 2,600oF, when the steel is austenitic, the hydrogen solubility is about .0001%. When the metallurgical structure is ferrite at 1,600oF, the solubility of hydrogen falls to about .00025%, and at a normal 70oF, .0002%. Because weld deposit hydrogen content from standard electrodes runs from .0001 to .002%, there is a significant risk of generating sufficient levels of hydrogen to supersaturate the molten weld from the core wire alone. The operator must reduce the available hydrogen in the coating for quality welds.
When steel is heated above its critical temperature (the point of temperature where there is a transformation from one metallurgical phase to another phase) and fully austenitic is cooled slowly it converts to a hard brittle martensitic structure. Cooled rapidly enough the austenite will not transform into martensite. The retained austenite now changes very slowly to martensite at temperatures from 400oF to room temperature. During the delayed transformation, the metal microcracks and fissures. If other stresses are present, cracking becomes aggravated and is easily detected. The defect may appear in the weld, at the weld interface, or in the parent metal, depending on how the hydrogen moves or where it becomes trapped.
Besides eliminating stress raisers, other precautions include reducing the retained austenite through carbon control, cold-working and holding the heat treating temperatures to close limits. Other defects, such as porosity, inclusions and notches should be eliminated, as they exacerbate hydrogen effects. It is not known for sure whether hydrogen causes porosity, but it does influence the amount of porosity in the weld.
Preventing hydrogen embrittlement is critical. Detecting a defect is difficult and frequently found only after the weld is put into service. Like cancer it grows and worsens with time. High strength steels, depending on high carbon content or low martensitic transformation properties, demand close watch for the possibilities of hydrogen absorption during welding. You cannot engineer or design the hydrogen from the weld. Heat drives out moisture, so turn on the heat.
Preventive Procedures
The easiest of all precautions to ensure a good weld is to avoid hydrogen forming compounds. Moisture, the most abundant and easiest to eliminate, is driven from a rod's coating by using high heat. New electrodes are normally dried and sealed at the factory and under ordinary conditions, are suitable for use straight from the can. However, to eliminate the chance of getting a defective can with moisture, put the electrodes in the oven.
Electrode drying ovens come in sizes holding from 15 to 1,100 lbs. and with temperature controls to 1000oF. The smaller ovens are portable, making it easy and convenient for the shop or field.
Rules for Drying Electrodes
Research has established enough information for the electrode manufacturer to recognize the importance of controlling the moisture in coatings. Most producers of electrodes and fluxes recommend procedures for heating their electrodes. Follow them and you'll be safe.
If you suspect that your electrodes are wet, and don't have a makers recommendation, remove the electrodes from the container and heat to 800oF in a drying oven. Baking time depends on the electrode's moisture content.
There is some indication that baking at 1000oF for 10 minutes is better than 800oF for 4 hours; however, you are safer with the lower temperature and longer time. When you heat the electrodes to 1000oF you risk breakdown of the coating which may prove more harmful than the presence of hydrogen.
If your electrodes are wet or were exposed to high moisture for a long period, bake at 800oF depending on the weld quality needed. For meeting X-ray quality and high strength steel building code requirements, wet electrodes should be discarded.
Dry Flux, Too
Flux used for submerged arc welding is another source of hydrogen. Treat flux the same as electrode coatings, especially if high strength steel is to be submerged arc welded. Never reuse dirty or fused flux. If you know the flux contains moisture, heat to 800oF for one hour, then store in a holding oven at 250o to 300oF. It is a good idea to put new flux in a holding oven for storage.
Flux cored wire, with the flux ingredients in the hollow of the wire, must also be watched for hydrogen forming conditions. It is possible for rust to form inside the wire. When this happens it is too late to correct in an oven.
The electrode drying equipment is relatively inexpensive, and quality fabricators and welding operators would never think of leaving their ovens cold.
What Happens to Electrodes* Under Normal Shop Exposure Conditions?
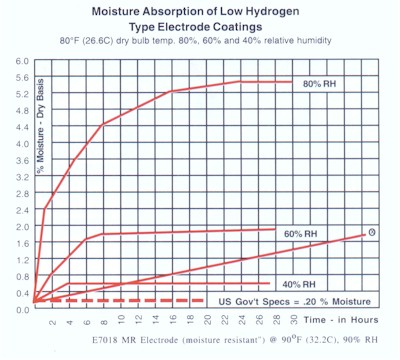
Within two hours at 80% relative humidity, rods may contain up to 13 times the allowable moisture content for U.S. Government & Nuclear Specifications. Within 24 hours, the rods may test up to 26 times the 0.2% allowed. Phoenix DryRod Ovens hold electrodes well within specified limits.
Dry Flux, Too... Flux used for submerged arc welding is another source for hydrogen. It is a good idea to put flux in a holding oven for storage. Treat flux the same as electrode coatings, especially if high strength steel is to be welded.
* Including, to a lesser extent, "moisture resistant" electrodes.